|
|
Profile
|
Delegates :
Yasuo Urata, M.Sc. |
|
Incorporated :
March 18 , 2004 |
Paid in Capital :
5072 Million yen |
Employees :
29 人 |
Address :
Toranomon Towers Office 10F 4-1-28 Toranomon, Minato-ku TOKYO
〒105-0001
|
TEL/FAX :
+81-5472-1578 / +81-5472-1488 |
URL:
www.oncolys.com |
Attachment :
|
Mission/Background :
Oncolys BioPharma is a public biopharmaceutical company in Japan mainly focusing on developing oncolytic viruses and seeking collaboration in immuno-oncology area.
|
|
Technology & Business
|
Our flagship oncolytic virus is OBP-301, currently phase 1/2 for hepatocellular carcinoma and esophageal cancer are ongoing and phase-2 for melanoma will start soon. OBP-301 showed synergistic efficacy with anti-PD-1 antibody, and thus Oncolys is very interested in collaborating with companies dedicated to immuno-oncology, such as developing immune-check-point inhibitors. Oncolys also develops cancer diagnostic to detect living circulating tumor cells (CTCs), both epithelial and mesenchimal phenotypes by using our proprietary virus.
In addition to above, Oncolys is developing HDAC inhibitor, anti HIV drug and anti HBV drug.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
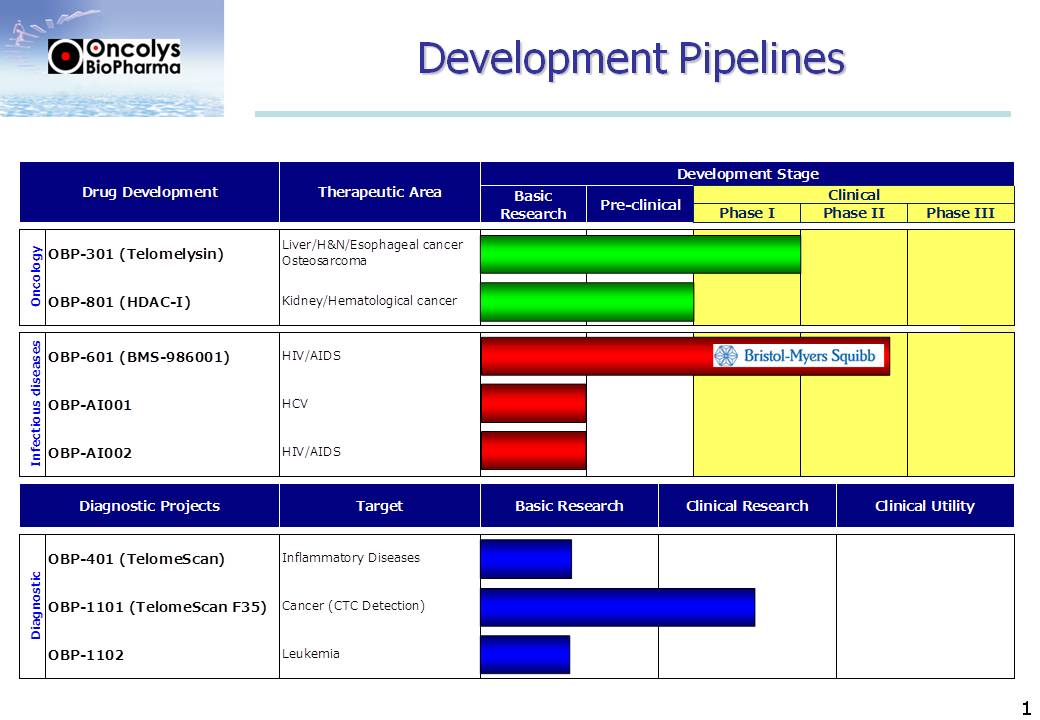
OBP-301 (Telomelysin)
|
Phase2
|
oncolytic adeno virus which is expected to induce immune response against cancer
|
Show effecacy and immuno-oncological response in Phase 2
|
OBP-601
|
Phase2
|
New anti HIV drug (NRTI)
|
Collaboration to start Phase-3
|
OBP-801
|
Phase1
|
Histone Deacetylase Inhibitors as anticancer agent
|
completion of Phase 1
|
TelomeScan
|
Discovery
|
tool for living CTC analysis
|
|
|
|
|
|
|
Highlights
|
Phase-1 for OBP-301 was completed in US and currently phase 1/2 for hepatocellular carcinoma and esophageal cancer are ongoing and phase-2 for melanoma will start soon.
We developed a novel system for detecting viable CTCs (circulating tumor cells) in the peripheral blood of cancer patients using a GFP-expressing tumor-specific replication-competent adenovirus (TelomeScan), which enable gene analysis without biopsy.
|
|
Alliance strategy
|
We are seeking out-license or collaboration opportunities in immuno-oncology area for OBP-301.
We hope to establish partnering with a company which can develop automation equipment, clinical testing company, or genetic testing company.
|
|
|