|
|
Profile
|
Delegates :
Hiroshi Nagabukuro |
|
Incorporated :
July 1 , 2018 |
Paid in Capital :
Million yen |
Employees :
6 人 |
Address :
24-8, Yamashita-cho, Naka-ku, Yokohama Kanagawa Japan
〒231-0023
|
TEL/FAX :
+81-45-225-8858 / +81-45-225-8858 |
URL:
https://www.arthamther.com/en/ |
Attachment :
|
Mission/Background :
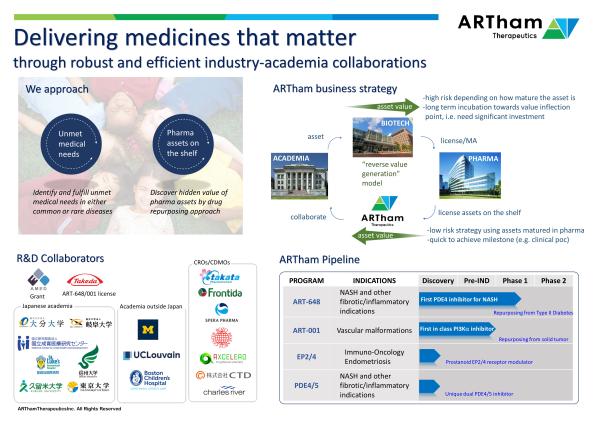
Deliver “medicines that matter” to improve patient health and contribute to enhanced quality of life. At ARTham Therapeutics, we will create value by developing innovative medicines that are disease modifying and improve patient health. |
|
Technology & Business
|
ARTham Therapeutics Inc., is a clinical stage biopharmaceutical company that will deliver ‘medicines that matter’ for patients. Our goal is to create innovative medicines that are disease modifying and satisfy significant unmet medical need. To deliver on cost and time efficiency, ARTham will run a virtual R&D operating model that seeks innovation through the best academic and business partners.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
ART-648
|
Phase1
|
First PDE4 inhibitor candidate for effective treatment of inflammatory skin diseases
|
Ph-2 POC clinical trial
|
ART-001
|
Phase1
|
First in Class PI3K inhibitor for the treatment of vascular malformations and overgrowth syndromes
|
Ph-2 POC clinical trial
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
1. Running a Ph-1 clinical trial of ART-648
2. Presented the preclinical data of ART-648 at International Liver Congress 2019
3. ART-648 has received a grant from AMED, Japanese Agency for Medical Research and Development (2020-2022)
4. Preparing a Ph-1 clinical trial of ART-001
5. ART-001 has received a grant from AMED, Japanese Agency for Medical Research and Development (2018-2020)
|
|
Alliance strategy
|
1. licensing OUT ARTham clinical program
2. Licensing IN new program(s), Discontinued program in pharmacos due to a business reason is high priority
3. Seeking CROs and/or CDMOs having excellent capability
|
|
|