|
|
Profile
|
Delegates :
Junichi Shimada, President & CEO |
|
Incorporated :
April 6 , 2001 |
Paid in Capital :
Million yen |
Employees :
60 人 |
Address :
Ichigo Kawasaki Bldg. 4F #404 1-2,Higashida-cho,Kawasaki-ku,Kawasaki City,Kanagawa Pref. Japan KANAGAWA
〒210-0005
|
TEL/FAX :
+81-44-201-6429 / +81-[44-201-6473 |
URL:
http://www.oncotherapy.co.jp/ |
Attachment :
|
Mission/Background :
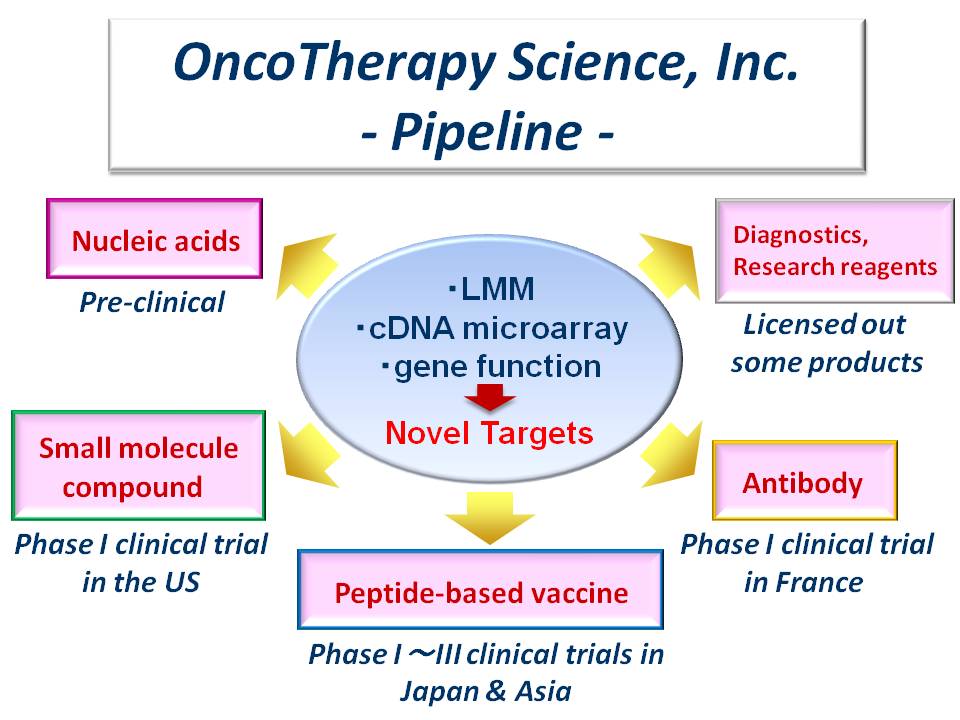
OncoTherapy Science, Inc. (OTS) provides innovative anti-cancer medicines with higher efficacy and a minimum risk of adverse events based on the comprehensive research on cancer genomics and biomedical analysis conducted by Human Genome Center, Institute of Medical Science, the University of Tokyo. OTS aims for the development of anti-cancer medicines such as peptide vaccines, antibodies, small molecular compounds and siRNAs, through our own research and/or collaboration with other pharmaceutical partners. |
|
Technology & Business
|
Our business area expands to a research for the development of new drugs with progress by continuous basic research. Based on the expression profiling with Laser Microbeam Microdissection and cDNA microarray system, we have identified many cancer-specific genes expressed in several types of cancers. Derived from those target genes, many epitope peptides restricted with HLA-A24 or -A2 were identified. Additionally, we promote the identification of epitope peptide restricted with other HLA-subtype for the cancer patients in Asia. In R&D of small molecule compounds targeting several “first-in-class” cancer-specific genes, we have obtained some compounds with high efficacy in vivo. We started phase I clinical trial of novel kinase inhibitor in the US. Also we are conducting clinical trial of novel antibody drug in France. We have the seeds of nucleic acid drug against cancer-specific genes, and we are working on the evaluation of in vivo efficacy.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
anti-Aβ antibody
|
Phase1
|
Alzheimer Disease
|
recrutment of joint research partner and
|
OTS167
|
Phase1
|
Solid tumor
|
Open public recruiting
|
OTSA101
|
Phase1
|
Synovial Sarcoma
|
Open public recruiting
|
OTSGC-A24
|
Phase1
|
|
|
|
|
|
|
|
Highlights
|
We have established the partnership with some companies to accelerate the clinical development of anti-cancer drug. We are performing the phase III clinical trial of C01 for pancreatic cancer (COMPETE-PC study) in Japan. Also the phase I/II clinical trial of OTSGC-A24 for gastric cancer is performed in Singapore, South Korea and Japan. Moreover, phase I clinical trial of novel kinase inhibitor in the US and phase I clinical trial of antibody drug in France have been started.
|
|
Alliance strategy
|
Peptide-based cancer vaccine has the potential of novel drug with minimum risk of adverse effect, and the clinical development of peptide vaccine has been started with some pharmaceuticals. We have some drug candidates, including peptide-based cancer vaccines, antibody drugs, small molecule compounds and nucleic acid drugs. We want to have the opportunity to meet with the new collaborator in order to provide novel anti-cancer medicines for the cancer patients. Any kinds of collaboration would be acceptable.
|
|
|