|
|
Profile
|
Delegates :
Dr. Yoshihiro Ito |
|
Incorporated :
April 5 , 2017 |
Paid in Capital :
7 Million yen |
Employees :
6 人 |
Address :
208,2-3-13,minami,Wako-city,Saitama SAITAMA
〒351-0104
|
TEL/FAX :
048-467-5811 / 048-467-5811 |
URL:
https://r-nanobio.co.jp/en/ |
Attachment :
|
Mission/Background :
We started as a RIKEN venture company. RIKEN is a National Research and Development Agency, is Japan's largest comprehensive research institution renowned for high-quality research in a diverse range of scientific disciplines.
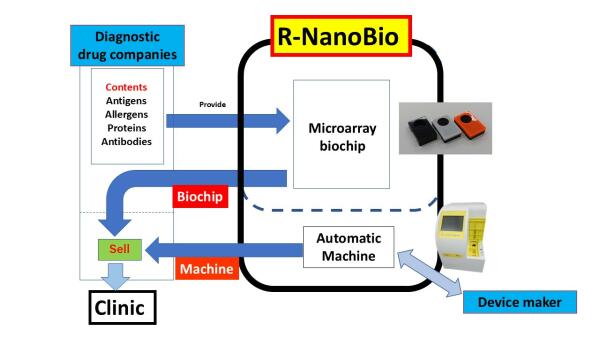
We have developed a new microarray preparation method using new special photo-reactive polymers. The microarray chips have covalently immobilized biopolymers, virus components, or cells and also provide high S/N ratio because of suppressing nonspecific protein adsorption on non-immobilized regions. In addition, we have developed an instrument that can automatically assay the interactions of molecules with the microarray-immobilized ones.
The world’s first compact microarray measurement platform was realized as DropScreen® which is commercially available from Nippon Chemiphar Co., Ltd. in Japan. The allergy detection kit is approved as an in vitro diagnostic and covered by Japanese Medical Insurance.
|
|
Technology & Business
|
A new microarray preparation method using new special photo-reactive polymers was already developed and used for DropScreen® which is commercially available from Nippon Chemiphar Co., Ltd. in Japan. The allergy detection system is approved as an in vitro diagnostic and covered by Japanese Medical Insurance.
DropScreen® detects 41 kinds of allergen-specific IgE within 30 min using 20 microliter of blood from fingertip. We would like to expand this allergy detection system worldwide.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
On-site allergy diagnosis system
|
Launched
|
Detects 41 allergen-specific IgEs within 30 min using 20 μl of fingertip blood with plans for global expansion.
|
Expansion of this allergy detection system worldwide
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
2019 October, DropScreen® is approved as an in vitro diagnostic and covered by Japanese Medical Insurance.
2020 February, DropScreen® is launched in Japan from Nippon Chemiphar Co., Ltd.
|
|
Hot news
|
2024 October, Exhibit at BioJapan.
|
|
Alliance strategy
|
Worldwide expansion of DropScreen®
Development of new diagnosis system using our platform for multiple detection (POCT) system.
|
|
|