|
|
Profile
|
Delegates :
YASUYUKI ISHII |
|
Incorporated :
March 3 , 2006 |
Paid in Capital :
535 Million yen |
Employees :
8 人 |
Address :
BRICKGATE NIHONBASHI 5F 35-3 Nihonbashi, Hakozaki-chou Chuou TOKYO
〒103-0025
|
TEL/FAX :
+81-3-5614-0007 / +81-3-5614-0008 |
URL:
http://www.regimmune.com |
Attachment :
 Chen_2017BBMT_RGI-2001_phase_study.pdf [ 1.3MiB ] Chen_2017BBMT_RGI-2001_phase_study.pdf [ 1.3MiB ] |
Mission/Background :
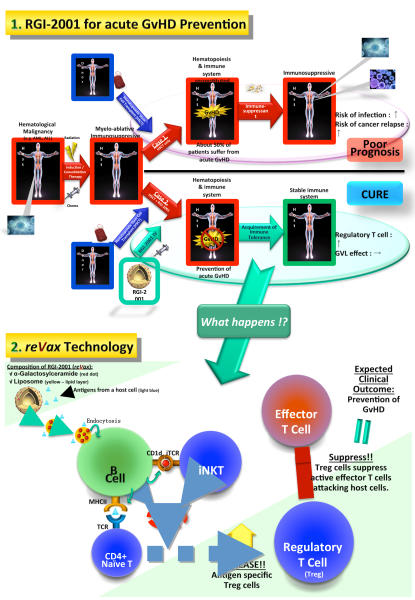
REGiMMUNE is a biotechnology company focused on the discovery, development and commercialization of immune regulatory therapeutics to treat life-threatening and debilitating conditions, including allergies, autoimmune diseases and transplantation. The company’s proprietary platform technology, reVax, induces immune tolerance in an antigen-specific manner through pharmacological induction of regulatory T (Treg) cells. |
|
Technology & Business
|
Using reVax technology, REGiMMUNE is developing RGI-2001, which may be the first drug in the class of Treg-inducing agents. The company is also applying its reVax technology to develop a range of pipeline products, including its RGI-1000 series for allergy and its RGI-3100 series for type 1 diabetes. Additionally REGiMMUNE is developing products for IBD.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
RGI-2001
|
Phase2
|
Prevention of GvHD in Patients With Hematological Malignancies Undergoing AHSCT
|
The company is seeking pharmaceutical partnership opportunities for its products worldwide, exclusive of Japan.
|
RGI-3600
|
Discovery
|
A novel α-GalCe oral formulation drug for IBD therapy
|
Seeking pharmaceutical collaborators
|
RGI-3100
|
Discovery
|
Liposomal α-GalCer plus insulin for induction of antigen-specific Tregs to type 1 diabetes patients.
|
The company is seeking pharmaceutical partnership opportunities for its products worldwide, exclusive of Japan.
|
|
|
|
|
|
|
|
|
|
Highlights
|
The potential of a novel approach using RGI-2001 and suboptimal dosage of antibody for that blocks CD40:CD40L signaling as a powerful method to generate mixed chimerism in various animal models of bone transplantation was proved (Toshihito Hirai, Yasuyuki Ishii and et al. A Novel Approach Inducing Transplant Tolerance by Activated Invariant Natural Killer T Cells with Co-stimulatory Blockade. Am J Transplant 2014 & 2016)
|
|
Hot news
|
REGiMMUNE Co is going to seek pharmaceutical partnership opportunities for RGI-2001 in BIOJAPAN 2014.
|
|
Alliance strategy
|
The company is seeking pharmaceutical partnership opportunities for RGI-2001 worldwide, exclusive of Japan.
|
|
|