|
|
Profile
|
Delegates :
Kenichi Nagai, President and CEO |
|
Incorporated :
May 8 , 2003 |
Paid in Capital :
5427 Million yen Increased due to IPO and financing after IPO |
Employees :
42 人 |
Address :
2-2-4, Kojimachi, Chiyoda-ku TOKYO
〒102-0083
|
TEL/FAX :
+81-3-5840-7697 / +81-3-5840-7716 |
URL:
https://www.brightpathbio.com/english/index.html |
Attachment :
|
Mission/Background :
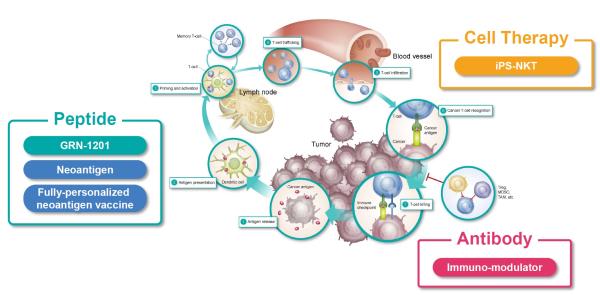
BrightPath Biotherapeutics, formerly known as GreenPeptide, is a Japan-based clinical-stage biopharmaceutical company focused on developing novel cancer immunotherapeutics. Our pipeline includes cancer peptide vaccine, rejuvenated cell therapy, and immuno-modulator antibodies. Our basic business model covers research and early clinical development, followed by out-licensing to pharmaceutical companies.
|
|
Technology & Business
|
BrightPath is targeting to research and development of promising drug candidate in the immuno-oncology area. We are conducting clinical studies of novel cancer peptide vaccine, Phase 2 study of GRN-1201 in the US.
Our next target is fully-personalized cancer vaccine, so we are conducting collaborative research to develop a methodology to identify a target set of neoantigens from the cancer mutations (mutated gene sequence data) with National Cancer Center Japan, Kanagawa Cancer Center, University of Tokyo, and Mie University.
Regarding cell therapy, we are conducting collaborative research of iPS-NKT cell therapy with RIKEN.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
GRN-1201 (HLA-A02 restricted cancer peptide vaccine)
|
Phase2
|
Indication: melanoma, non-small cell lung cancer (in combination with immune checkpoint inhibitor)
|
To acquire effective data in combination of an immune checkpoint antibody
|
iPS-NKT cell therapy
|
Preclinical
|
Indication: Head and neck cancer
|
To initiate investigator initiated clinical trial
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
(GRN-1201)
2015 Start of phase I for malignant melanoma in the U.S.
2017 Start of phase II in combination with immune checkpoint inhibitor for NSCLC in the U.S.
(iPS-NKT)
2018 Start of collaborative research with RIKEN
(Fully Personalized Neoantigen Vaccine)
2017 Start of collaborative research with National Cancer Center Japan
2018 Start of collaborative research with University of Tokyo and Kanagawa Cancer Center
Start of collaborative research with Mie University
|
|
Alliance strategy
|
Brightpath is seeking out-licensing partners regarding our promising pipeline and in-licensing/co-development partners to enhance our scientific basis in cancer immunotherapy.
|
|
|