|
|
Profile
|
Delegates :
Keiichi FUKUDA, MD/ PhD/FACC |
|
Incorporated :
November 30 , 2015 |
Paid in Capital :
Million yen |
Employees :
41 人 |
Address :
Seavans South Building 5F 1-2-3 Shibaura, Minato-Ku TOKYO
〒105-0023
|
TEL/FAX :
+81-3-6380-1068 / |
URL:
http://www.heartseed.jp/en/index.html |
Attachment :
|
Mission/Background :
Heart failure is a condition in which the heart's pumping function is impaired due to necrosis of the heart tissue caused by myocardial infarction or other causes. It is estimated that 1.3 million people suffer from heart failure in Japan. The lost heart tissue cannot be restored to its original state, and the only possibility for a cure is a heart transplant.
Dr. Fukuda was the first in the world to successfully induce differentiation of cardiac muscle cells from bone marrow mesenchymal stem cells, and has published numerous research results and obtained patents toward the realization of cardiac regenerative medicine. The company has also obtained a number of patents. Aiming for early clinical application of these results, he established Heartseed Inc. in 2015. With the mission of "Opening the door for cardiovascular disease treatment with regenerative medicine," we are working toward the practical application of regenerative medicine as soon as possible. |
|
Technology & Business
|
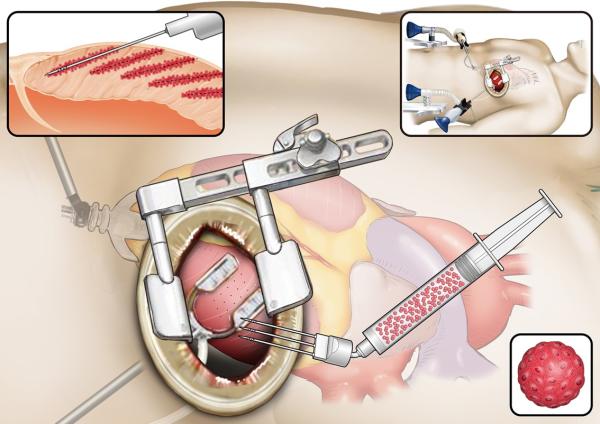
Our lead pipeline HS-001 is a spheroid of cardiomyocytes derived from allogeneic iPS cells and is intended for patients with severe heart failure insufficient response to existing therapies.
We are developing, in addition to paracrine effects, replenishes regenerated myocardium that contracts in synchronization with the remaining myocardium, thereby increasing the number of functioning myocardium itself.
To address the risks of tumorigenesis and arrhythmia in the realization of the therapy, we have established purification technology to eliminate undifferentiated iPS cells (patented in major countries) and technology to selectively create ventricular muscle.
We are developing open chest administration and catheter administration of the spheroids for clinical application, and the former is undergoing clinical trials in Japan.
Furthermore, in 2021, we concluded a worldwide collaborative license agreement with Novo Nordisk for HS-001, and secured the development of the product for the global market.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
HS-001, allogeneic iPSC-derived purified cardimyocytes for heart failure
|
Phase1/2
|
First-in-class ventricular specific cardiomyocytes that provide direct contractile function.
|
Obtain approval as soon as possible.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
The initial results of the LAPiS trial in 2023 have been reported by investigative sites at major academic conferences and other events, attracting a high level of attention. We have also announced a license-out agreement for the removal technology of undifferentiated iPS cells.
In 2022, the company received the Most Promising Pipeline Award at the Asia Pacific CGT Excellence Awards, and in 2023, it won the Grand Prix in the startup category at the 4th IP BASE AWARD hosted by the Japan Patent Office.
|
|
Alliance strategy
|
We are hoping for alliance opportunities with a focus on the following projects
Out-licensing deals: Undifferentiated iPS cell removal technology
In-license deals: therapeutic drugs (regardless of modality) or cellular therapeutics (regardless of disease) in the field of cardiac disease.
|
|
|