|
|
Profile
|
Delegates :
President and CEO Koichiro Tsuji |
|
Incorporated :
April 23 , 2003 |
Paid in Capital :
752 Million yen as of July 11, 2016 |
Employees :
23 人 |
Address :
Hiroshima Sangyou Bunka Center Bldg., 11F 16-35 , Hijiyamahonmachi , Minami-ku , Hiroshima City HIROSHIMA
〒732-0816
|
TEL/FAX :
+81-82-250-3138 / +81-82-250-3148 |
URL:
http://www.twocells.com/ |
Attachment :
|
Mission/Background :
TWO CELLS, Co., Ltd was founded as Bio-Venture in April, 2003, spun off Hiroshima University .We have been working in the development of mesenchymal stem cell(MSC) culture technology and of tools for MSC therapy. Our major pipelines of regenerative medicine are serum-free chemically defined media for MSCs and "gMSC1" a Regenerative Cellular Medicine for Chondrogenesis in the Knee. |
|
Technology & Business
|
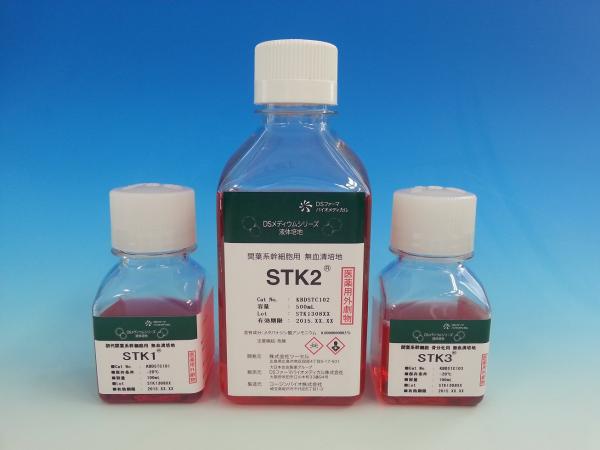
We developed serum-free chemically defined medium STK2, STK1 (for primary MSC ) , STK3(for Bone differentiation), as non FBS medium, and launched in 2008 in order. The benefits of serum-free chemically defined media are the avoid of risks such as BSE pollution and other infectious diseases. Moreover, the qualities of STK1,STK2 and ,STK3 are stable to the cultures of MSCs derived from various tissues.Anotherone, gMSC1 is a tissue-engineered medical product currently developed by TWO CELLS and was created for the purpose of regenerative chondrogenesis using synovium-derived mesenchymal stem cell (MSC). This product is a scaffold-free allogeneic 3D artificial tissue of MSC provided by TWO CELLS with its own technologies and serum-free medium (STK1 and STK2), which is expected to provide an effective treatment for cartilage regeneration.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
serum-free media STK2,STK1,STK3
|
Launched
|
The benefits of serum-free chemically defined media are the avoid of risks such as BSE pollution and other infectious diseases.
|
world wide licensing of STK media developmentfor research and clinical uses
|
"gMSC1," a Regenerative Cellular Medicine for Chondrogenesis in the Knee
|
Preclinical
|
gMSC1 is a tissue-engineered medical product currently developed by TWO CELLS.
|
conduct clinical studies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
Chugai Pharmaceutical Co., Ltd. and TWO CELLS Co., Ltd. announced that on April 25, 2016, both companies concluded a licensing agreement regarding gMSC1, a regenerative cellular medicine for chondrogenesis in the knee, created by TWO CELLS.
|
|
Hot news
|
Chugai Pharmaceutical Co., Ltd. and TWO CELLS Co., Ltd. announced that on April 25, 2016, both companies concluded a licensing agreement regarding gMSC1, a regenerative cellular medicine for chondrogenesis in the knee, created by TWO CELLS.
|
|
Alliance strategy
|
world wide licensing of STK series.
|
|
|