|
|
Profile
|
Delegates :
Prof. Kanji Takada, Ph. D. |
|
Incorporated :
August 1 , 2001 |
Paid in Capital :
10 Million yen |
Employees :
3 人 |
Address :
Shimizu-cho 389, Nakagyo-ku, Kyoto city KYOTO
〒604-0874
|
TEL/FAX :
+81-75-746-2160 / +81-75-746-2160 |
URL:
http://www.bioserentach.co.jp/e-index.html |
Attachment :
|
Mission/Background :
BioSerenTach (BST), technology-oriented company, was established in 2001. Dr. Takada, the chairman, was a professor of Kyoto Pharmaceutical University to accelerate the development of his DDSs. Based on the IPs in 3 area, BST collaborates to develop new oral and percutaneous DDS products with pharmaceutical companies having API. Our role is up to preclinical trials, and trials after phase I will be performed by pharmaceutical companies. Using API, BST prepares test DDS formulation and performs formulation tests. Animal study is performed in collaboration with university laboratories. |
|
Technology & Business
|
BST provide the following two new oral and percutaneous DDSs that enable the absorption of low-membrane permeable drugs such as peptides and nucleic acids.
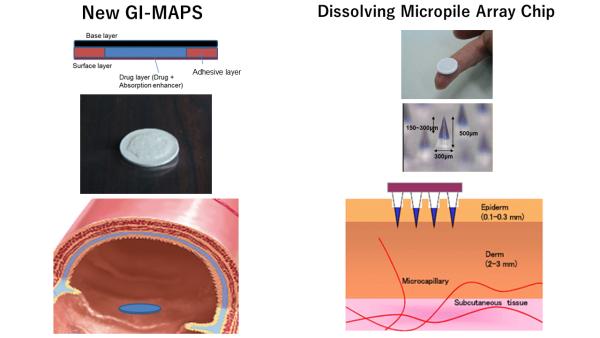
1. Gastrointestinal mucoadhesive patch system (GI-MAPS)
GI-MAPS is a tablet consisting of three parts, (1) adhesion site controlling surface layer, (2) drug layer containing absorption enhancer, (3) water-insoluble base layer preventing the invasion of hydrolase, and improves the bioavailability (BA) of the drug.
Example: EPO 7.2%, G-CSF 23%
2. Dissolving Micropiles (DMs)
DM is a chip-like formulation molded into a needle-like kneading of a drug with the base made of water-soluble thread-forming polymer such as chondroitin sulfate, dextran and hyaluronic acid. DM physically breaks the skin barrier and drug is delivered to the epidermis layer of the skin. 100% BA is achieved.
Example: Growth hormone 100%, Insulin 98%, Heparin 100%, Desmopressin 95%, Leuprolide 99%.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
GI-MAPS
|
Preclinical
|
Preparation of test GI-MAPS, Pharmaceutical evaluation, Animal study
|
Matching with best in the class compound, Global expansion,
|
Dissolving micropiles (DMs)
|
Phase2
|
Preparation of test DM chip, Pharmaceutical evaluation, Animal study. Doctor-directed investigator-initiated clinical phase II
|
Matching with clients, Global expansion
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
・Improved new GI−MAPS patent has been completed.
・GI−MAPS related oral peptide DDS:Novo Nordisk filed for FDA approval of oral semaglutide, which would be the first glucagon-like peptide-1 (GLP-1) receptor agonist in a pill form which contains 4mg semaglutide and 300mg absorption enhancer, SNAC. BA of semaglutide from the stomach is 1%.
・Patent on new polymer base having strong adjuvant effect has been completed.
|
|
Alliance strategy
|
・BST is looking for a co-developer on b-FGF DM array chip for the regeneration of the skin, anti-aging.
・Looking for pharmaceutical companies to co-develop GI−MAPS for oral delivery of macromolecular drugs.
・Looking for licensee
・Looking for companies to co-develop DMs as skin vaccine.
|
|
|