|
|
Profile
|
Delegates :
Masuhiro Yoshitake,Hitoshi Endou |
|
Incorporated :
December 26 , 2005 |
Paid in Capital :
Million yen |
Employees :
7 人 |
Address :
1-308 Leading Venture Plaza, 75-1 Onocho, Tsurumi-Ku, Yokohama KANAGAWA
〒2300046
|
TEL/FAX :
45-506-1155 / 45-506-1156 |
URL:
http://www.j-pharma.com |
Attachment :
|
Mission/Background :
J-Pharma Co. Ltd.,which was founded in 2005 by Dr. Hitoshi Endou, the emeritus professor of Kyorin University, is a Japanese biopharmaceutical company with significant expertise in transporter target drug research and development. We enriches the product pipeline by Dr. Endou's original research compounds, through new molecular entity research in collaboration with universities both in Japan and USA. |
|
Technology & Business
|
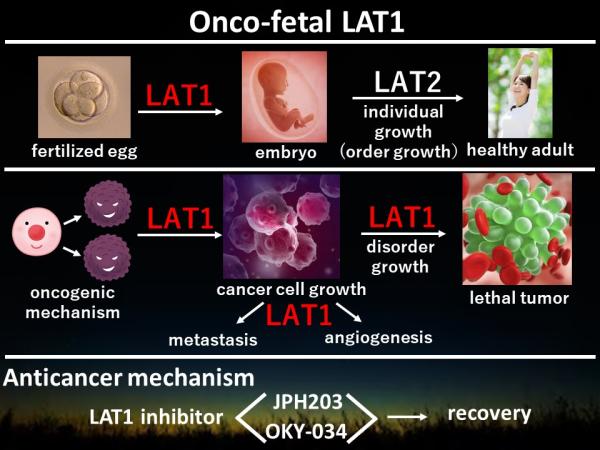
LAT1 has been patented by J-Pharma. J-Pharma has developed a variety of LAT1-specific inhibitors which inhibit essential amino acids (i.e. like leucine, isoleucine, methionine, phenylalanine and valine) uptake into cancer cells. LAT1 selective inhibition stimulates apoptosis of cancer cells via the so called 'starve-out' strategy, a specific LAT1-mediated cell signaling pathway. LAT1 is an Onco-fetal membrane protein highly expressed in transformed cancer cells. LAT1 is found in a few normal tissues like bone marrow, reproductive organs and brain. LAT2 is a homolog of LAT1 and expressed ubiquitously in normal tissues, but not expressed in cancer cells.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
JPH203
|
Phase2
|
Anti cancer drug
|
Complete clinical test in 2021
|
OKY-034
|
Phase1/2
|
Anti cancer drug
|
Complete clinical test in 2019
|
NKO028
|
Preclinical
|
PET/CT compounds
|
acquire POC in 2019
|
NC2700
|
Preclinical
|
Anti-hyperuricemic agent
|
licensing in 2019
|
|
|
|
|
|
Highlights
|
JPH203: Phase II clinical test was started in September 2018.
OKY034: Phase I/IIa clinical test was started in March 2019.
|
|
Alliance strategy
|
Product for license-out; JPH 203 and OKY-034 anti cancer drug, NKO028 PET/CT compounds, NC2700 Anti-hyperuricemic agent.
Transporter intellectual property for license-out LAT1, LAT3, URAT1, OAT1,2,3,4
|
|
|