|
|
Profile
|
Delegates :
Hitoshi SHIOMI |
|
Incorporated :
August 17 , 2007 |
Paid in Capital :
459 Million yen |
Employees :
8 人 |
Address :
OkayamaYanagimachi Bldg., 4F 1-12-1, Yanagimachi, Kita-ku, Okayama OKAYAMA
〒7000904
|
TEL/FAX :
+81-86-238-7848 / +81-86-238-7848 |
URL:
https://www.mt-gene.com/en/ |
Attachment :
|
Mission/Background :
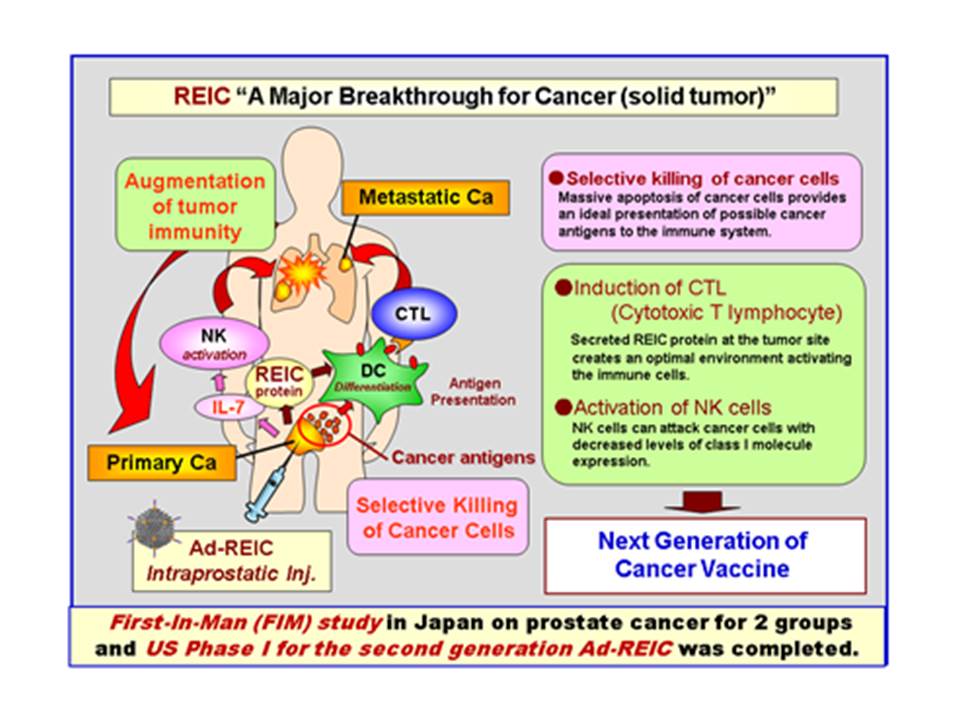
Momotaro-Gene Inc. is a biotech company spinning out of Okayama University. The company mission is to bridge technological seeds from academia to the industry or collaboration with the industry to launch new drug to the market. Our first technology is REIC/Dkk-3 gene therapy. This gene is isolated and cloned at Okayama University by Prof. Namba and his team. When he did the immortalized cell research, he found the expression of that specific gene very much reduced and named it REIC (Reduced Expression in Immortalized Cells). This gene is a tumor suppressor gene whose expression is reduced in most of the tumors.
Later, Prof. Kumon, former Chair of Urology Dept. there has led its clinical development and established Momotaro-Gene Inc., promoting the clinical study in Japan and clinical trial in the US both on prostate cancer to establish the POC of Ad-REIC gene therapy to solid tumor. We are now promoting clinical development for other indications such as liver cancer and malignant mesothelioma. |
|
Technology & Business
|
Ad-REIC gene therapy has the promising anti-cancer effect with minimally invasive side effect (only temporary fever) by causing cancer specific apoptosis via ER stress and enhancing tumor immunological effect with REIC protein. Its POC for the first- generation product was established by the Okayama University clinical study on the 26 prostate cancer patients. The several-time higher effective second-generation product, Ad-SGE-REIC, also completed its Phase I study in the US with 12 patients with the same side effect and several-time higher efficacy. In Japan, Kyorin Pharmaceutical started Ad-SGE-REIC Phase I clinical trial for mesothelioma in September, 2015. We have also started its Phase I clinical for liver cancer at Okayama University Hospital from July, 2017 and for glioma from July 2019. In the US, we have been doing its Phase IIa trial for early prostate cancer since July, 2016. At Baylor College of Medicine, we will start P2 combination therapy with anti Pd-1 Ab for mesothelioma in August, 2019.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
Ad-SGE-REIC
|
Phase2
|
Ad-REIC gene therapy induces tumor specific apoptosis via ER stress and enhances cancer immunity via REIC protein
|
Entering Phase II combination therapy with anti PD-1 Ab(US), Phase I/II trial for glioma (JP, monotherapy)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
In the US, we entered into Ad-SGE-REIC Phase IIa trial for prostate cancer in July, 2016. FDA granted its orphan status for mesothelioma. We will start Phase 2 combination therapy with a PD-1 Ab for mesothelioma at Baylor College of Medicine in August, 2019.
In Japan, Kyorin Pharmaceutical started its malignant mesothelioma clinical trial in September, 2015. We have also started its Phase I clinical trial for liver cancer at Okayama University Hospital from July, 2017 and for glioma from July, 2019.
|
|
Hot news
|
We will start Phase II combination therapy with a PD-1 Ab at Baylor College of Medicine in August, 2019.
|
|
Alliance strategy
|
We would like to have a license deal with the pharmaceutical or bio industry for the US/EU right of Ad-SGE-REIC
|
|
|