|
|
Profile
|
Delegates :
Tetsu Uchigasaki |
|
Incorporated :
June 29 , 2016 |
Paid in Capital :
Million yen |
Employees :
5 人 |
Address :
TOKYO
〒100-0004
|
TEL/FAX :
+81-3-4243-8654 / |
URL:
http://curadim.co.jp/en/ |
Attachment :
|
Mission/Background :
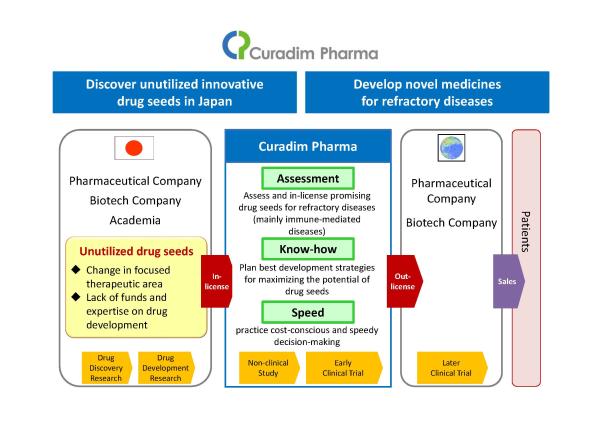
We, Curadim Pharma, are a pipeline-driven biotech company established with the mission of “Discover unutilized innovative drug seeds in Japan” and “Develop novel medicines for refractory diseases.” We discover unutilized innovative drug seeds in Japan and focuses on conducting the early stage of drug development (non-clinical studies and early-phase clinical trials). By developing novel medicines originating in Japan, we create value in treatments for refractory diseases with high unmet medical needs. |
|
Technology & Business
|
We aim to discover unutilized innovative drug seeds in Japan and enhance the flow of drug development through implementation of our business model as a company specialized in conducting the early stage of drug development (non-clinical studies and early-phase clinical trials).
(1) Assess and in-license drug seeds for refractory diseases (mainly immune-mediated diseases) with high unmet medical needs which are promising, but are unused in Japanese pharmaceuticals, biotech companies, and academia.
(2) Plan and practice best development strategies for maximizing the potential of drug seeds by experienced and professional team.
(3) Out-license to a global major pharmaceutical company after quick acquisition of POC through cost consciousness and speedy decision-making in management.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
CP1050 (S1P receptor modulator)
|
Phase1
|
Indication: Autoimmune diseases such as MS Mechanism: S1P receptor modulator
|
Completion of Phase 1 clinical trial
|
CP2090 (LPA1 selective antagonist)
|
Preclinical
|
Indication: Fibrotic diseases such as IPF and NASH Mechanism: LPA1 selective antagonist
|
Completion of GLP non-clinical studies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
CP1050:Phase 1 clinical trial was started in the UK.
CP2090:License agreement was concluded.
|
|
Alliance strategy
|
Our business model is to out-license the pipelines to a global major pharmaceutical companyafter quick acquisition of POC. Please contact us if you are interested in our pipelines.
We also focus on in-licensing of unutilized drug seeds in Japan. We look forward to receiving information on your promising drug candidates for refractory diseases (mainly immune-mediated diseases) with high unmet medical needs.
|
|
|