|
|
Profile
|
Delegates :
Shotai Kobayashi |
|
Incorporated :
January 5 , 2016 |
Paid in Capital :
335 Million yen |
Employees :
5 人 |
Address :
Izumo-shi, Enya-cho, 89-1 SHIMANE
〒693-8501
|
TEL/FAX :
0853-25-3033 / 0853-25-3032 |
URL:
http://www.purec.jp/ |
Attachment :
 Purecパンフレット3-081616Eng_ver_(高画質).pdf [ 1.5MiB ] Purecパンフレット3-081616Eng_ver_(高画質).pdf [ 1.5MiB ] |
Mission/Background :
Based on the separation method to isolate high-quality mesenchymal stem cell from bone marrow, which was established by Dr. Matsuzaki, professor in the medical department of Shimane University, PuREC was established in January, 2016, supported by Shimane University and San-in Godo Bank in terms of management and finance.
We provide highly purified human mesenchymal cell (REC:Rapidly Expanding Cells) for research use and are establishing GMP-grade, stable production system for the application to transplantation therapy.
We are in discussion with Japanese regulatry authority (PMDA) ,regarding the clinival development of REC for the treatment of hypophosphatasia, one of lethal Congenital Skeletal Diseases. |
|
Technology & Business
|
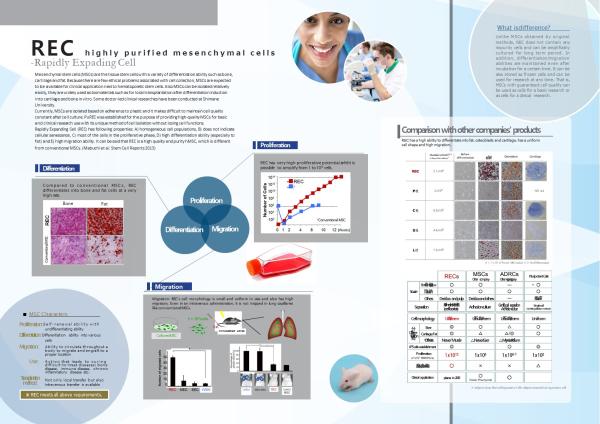
Currently available MSCs are isolated based on adherence to plastic dish but are inevitably contaminated by cells without differentiation ability, resulting in difficulty to maintain cell quality constant. In addition, during culture, they lose migration ability which they have when they are in bone marrow.
PuREC established highly effective method to isolate MSC by using anti-LNGFR(CD271) and anti-Thy-1(CD90) antibodies. It is confirmed that MSC obtained by this method(REC: Rapidly Expanding Cells) has high homogeneity and excellent ability to proliferate, differentiate and migrate.
In November, 2017, a research proposal to develop REC as a transplantation therapy for the treatment of Hypophosphatasia, was adopted by AMED’s program for strategic promotion of translational clinical trial(Seeds B).In August, 2018 a research proposal to establish AI-based quality control system of REC was adopted by NEDO’s supporting program for next generation artificial intelligence and robotics technologies
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
Highly purified human mesenchymal cell from bone marrow (REC:Rapidly Expanding Cells)
|
Preclinical
|
Clinical development of REC as a transplantaion therapy for the treatment of Hypophosphatasia
|
Commencement of invetigator initiated clinical trial in 2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
On December 6th, 2018, the patent entitled "METHOD FOR EVALUATING QUALITY OF HUMAN MESENCHYMAL STEM CELL, AND
MONOCLONAL ANTIBODY FOR USE IN SAID METHOD" was issued in Japan.
Now we are in discussions with Japanese regulatory authority(PMDA ) regarding the clinival development of REC for the treatment of hypophosphatasia, one of lethal Congenital Skeletal Diseases
|
|
Hot news
|
This June, we financed 580 million yen by allocation of new shares to a third party.
|
|
Alliance strategy
|
PuREC is seeking partners for opening new frontiers of clinical application of MSC.
Please contact us when you want to start your research using our cell, or when you want to start your research to establish novel therapy using your device or seeds in combination with with our cell
|
|
|