|
|
Profile
|
Delegates :
Satoshi Sato |
|
Incorporated :
December 6 , 2006 |
Paid in Capital :
6 Million yen |
Employees :
1 人 |
Address :
Okayama Research Park Incubation Center 5303 Haga Kita-ku Okayama City OKAYAMA
〒701-1221
|
TEL/FAX :
+81-86-286-9507 / +81-86-586-9508 |
URL:
http://www.nomadicbio.com/en |
Attachment :
|
Mission/Background :
Nomadic Bioscience Co., Ltd. was established by Satoshi Sato, currently CEO, on December 2006 after he was awarded a prize in the Okayama-prefecture venture business plan contest. Our business includes research and development of proteins for industrial use, and production and distribution of reagents for research use. |
|
Technology & Business
|
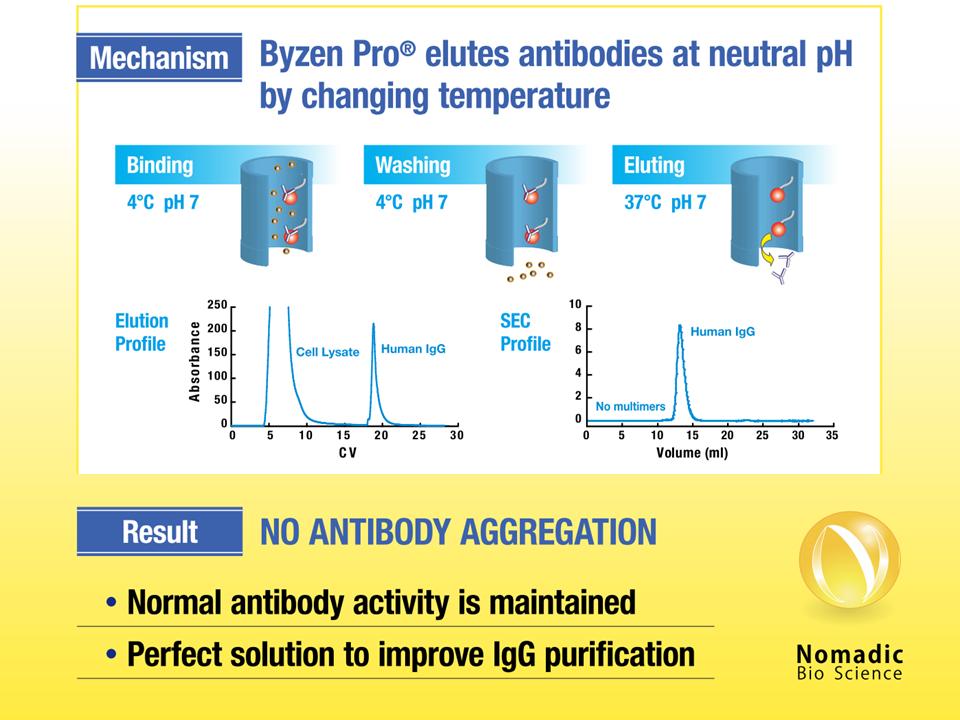
We bring innovation in antibody purification. Our Protein A resin (Byzen Pro®) reduces the manufacturing cost of antibody drug by eliminating risk of antibody aggregation. The engineered Protein A avoids the use of acid and elutes antibody at neutral pH by simply changing temperature, significantly increasing a recovery rate of antibody. We are seeking clients that wish to test the breakthrough technology.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
Temperature-responsive Protein A resin, Byzen Pro
|
Launched
|
Antibody can be eluted at neutral pH by changing temperature, eliminating risk of aggregation and decreased activity.
|
We are seeking clients that wish to test the breakthrough Protein A resin.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Highlights
|
1) We recently have entered into a supplier contract with a major US pharmaceutical company.
2) We have started a joint project with a major US pharmaceutical company for developing a high-throughput format of Byzen Pro.
|
|
Hot news
|
"Byzen Pro" (5 mL/25 mL) is now available from Sigma-Aldrich as a research-grade reagent. It is also available from a Japanese distributor Funakoshi Co., Ltd.
|
|
Alliance strategy
|
1) We are seeking clients that wish to test the breakthrough Protein A resin.
2) We are also looking for a partner, in particular resin companies, for GMP production.
|
|
|