|
|
Profile
|
Delegates :
Yonehiro Matsumura |
|
Incorporated :
January 15 , 2002 |
Paid in Capital :
6560 Million yen Listed on Tokyo Stock Exchange (Mothers) since February 2013 |
Employees :
26 人 |
Address :
431-7 Nishiyama Higashikagawa-city, Kagawa, Japan KAGAWA
〒769-2712
|
TEL/FAX :
+81-879-23-3071 / +81-879-23-3072 |
URL:
http://www.medrx.co.jp/english/index.html |
Attachment :
|
Mission/Background :
MEDRx is a pharmaceutical venture company capitalizing on unique transdermal technologies.
ILTS(R) : Ionic Liquid Transdermal System
NCTS(R) : Nano-sized Colloid Transdermal System
AMRTS : Abuse and Misuse Resistant Transdermal System
KENZAN : Micro Needle Array |
|
Technology & Business
|
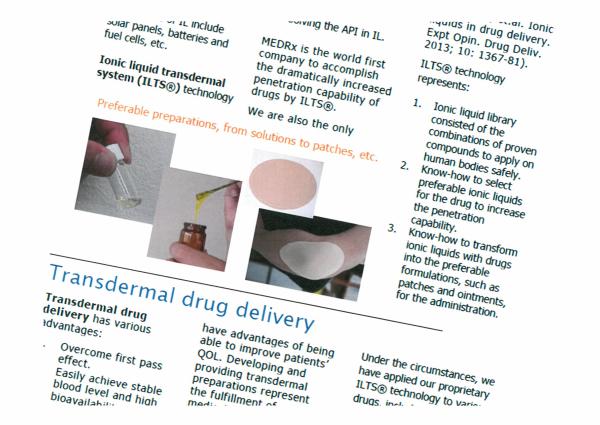
Ionic liquid transdermal system (ILTS(R)) technology enables to aquire adequate solbility of various APIs through:
- Forming ionic liqid with the API, or
- Solving the API in ionic liquid.
MEDRx is the world first company to accomplish the dramatically increased penetration capability of drugs by ILTS(R). We are also the only company to have run clinical trials on the efficacy of API-IL (Rogers RD, et.al. Ionic liquids in drug delivery. Expt Opin. Drug Deliv. 2013; 10: 1367-81).
ILTS(R) technology represents:
1. Ionic liquid library consisted of the combinations of proven compounds to apply on the human bodies safely.
2. Know-how to select preferable ionic liquids for the drug to increase the penetration capability.
3. Know-how to transform ionic liquids with drugs into the preferable formulations, such as patches and ointments, for the administration.
ILTS(R) patent portfolios cover preparations for wide range of APIs.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
MRX-4TZT, Tizanidine transdermal patch for the management of Spasticity
|
Phase1
|
Signed a worldwide licensing agreement (except for East Asia) with Cipla Tech
|
Start of phase 2 study in US
|
MRX-5LBT
|
Phase3
|
Lidocaine topical patch for PHN
|
NDA filing in US in 2020
|
MRX-1OXT
|
Phase1
|
Oxycodone transdermal patch for the chronic pain management
|
Start of phase 1b (repeated PK) study in US
|
MRX-7MLL
|
Preclinical
|
memantine transdermal patch, 3 days or 1 week patch
|
Start of phase 1 study in US
|
|
|
|
|
|
Highlights
|
In April 2017,
MEDRx has signed a worldwide licensing agreement (except for East Asia) with Cipla USA Inc. to further develop and commercialize MRX-4TZT, a Tizanidine patch for the management of Spasticity.
In August 2018,
MEDRx announced a Technology License Agreement with Takeda Pharmaceutical Company Limited (Takeda) to support the development of selected Takeda pipeline compounds in a focused therapeutic area with its proprietary technology for transdermal drug delivery.
|
|
Alliance strategy
|
We are now seeking partners who are willing to in-license MRX-5LBT (lidocaine patch) , MRX-1OXT (oxycodone patch) or MRX-7MLL (Memantine patch) to feed pipeline.
We are also seeking for counterparts who are willing to in-license the ILTS(R) / NCTS(R) technology and/or to collaborate in developing a novel transdermal formulation.
We are also seeking for collaboration partners regarding Micro Needle Array.
|
|
|