|
|
Profile
|
Delegates :
Kazuhiro Matsuda |
|
Incorporated :
January 5 , 2005 |
Paid in Capital :
202 Million yen |
Employees :
3 人 |
Address :
2-1-3-1103 Fukasawa, Setagaya-ku TOKYO
〒158-0081
|
TEL/FAX :
090-8486-5727 / 03-3705-5230 |
URL:
https://en.mbiotechnology.com/ |
Attachment :
|
Mission/Background :
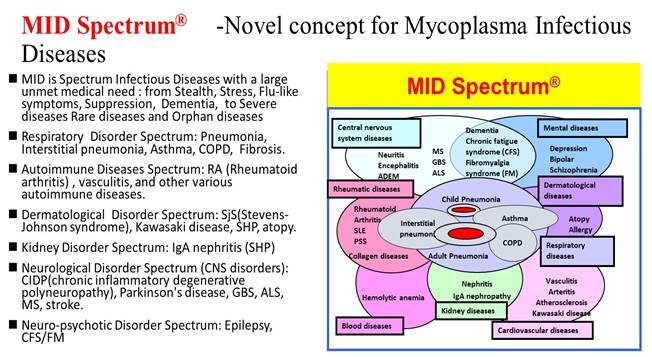
M Bio Technology is a world leading company focused on the discovery and development of innovative therapeutic strategy for Mycoplasma Infectious Diseases (MID). MID Spectrum(R) includes diseases with large, unmet medical needs - from acute to chronic hard-to-treat diseases and rare and orphan diseases. With the innovative diagnostic system MID Prism(R), we develope innovative vaccine, diagnostics, precision therapy for MID. Because MID is transmittable disease, it is an important target to control and prevention.
|
|
Technology & Business
|
We propose preventive and precision medicine as a solution to MID. How accurately early discovery and diagnosis is an important turning point for proper medical care. An innovative diagnostic system (MID Prism(R)) provides a high-quality reliable biomarker of MID. Vaccines and antibody drugs for MID are also covered by patents. Big data of information based on MID Prism(R), contribution to infectious disease countermeasures and public health and health welfare by the MID surveillance system ICT/A I (MID Navigator(R)).
MID Prism(R) has made it possible to measure the precise amounts of each IgM, IgG, and IgA antibodies. Glycolipid antigens of the cell membrane of mycoplasma are believed to play an important role in the immune response. Natural killer T (NKT) immune cells can distinguish substrates have a strong immunomodulatory function. This special ability of NKT cells has great potential for treatment of incurable autoimmune diseases as well as cancer and infection immunotherapy.
|
|
Products & Service
|
Products & Service Name
|
Stage
|
Outline
|
Milestone
|
MID Vaccines(TM)
|
Preclinical
|
Vaccine for Mycoplasma pneumoniae and Mycoplasma fermentans
|
Preclinical〜Phase1/2/3, seeking partner and funding
|
MID Antibodies(TM)
|
Preclinical
|
Therapeutic antibodies for M. pneumoniae and M. fermentans
|
Preclinical〜Phase1/2/3, seeking partner and funding
|
MID Prism(R)
|
Phase2/3
|
Innovative diagnostics for M. pneumoniae and M. fermentans
|
Partnering, Service, Networking
|
MID Navigator(R)
|
Discovery
|
Software to support the treatment of MID
|
Partnering, Service, Networking
|
MID Precision Medicine(TM)
|
Launched
|
Precision preventive medicine for MID
|
Partnering, Service, Networking
|
|
Highlights
|
Memorandum of understanding with major pharmaceutical companies on Mycoplasma pneumoniae vaccine prepares for preclinical and clinical trials
JETRO Global Acceleration Hub Adopts Drug Discovery Venture for Overseas Expansion Support Business
GLYCEROGLYCOLIPID ANTIGEN OF MYCOPLASMA PNEUMONIAE: WO2007145362A1
VACCINE FOR MYCOPLASMA INFECTION: WO2010140377A1
2019 BIO International Convention
Exhibited at Japan Pavilion (sponsored by JETRO)
|
|
Hot news
|
New Value Creation Exhibition 2018 New Value Creation Award
2019 JETRO Global Acceleration Hub Adopted drug discovery venture targeted by overseas expansion support business
|
|
Alliance strategy
|
M Bio Technology Inc. is seeking for partners of new therapeutic drugs or re-positioning with pharmaceutical industries using the innovative mycoplasma companion diagnostics.
M Bio Technology Inc. is seeking partnering opportunities to create a global network for advanced precision medicine.
Also, it is necessary to propose the importance worldwide including WHO, because MID is transmittable infectious diseases which is a cause of various incredible diseases.
|
|
|